19A135
A review of patients started on Janus Kinase inhibitor (JAKi) in University Hospital Waterford
Author(s)
Dr. Asif Munir Dr. Caire Sheehy
Department(s)/Institutions
Department of Rheumatology, University Hospital Waterford
Introduction
In 2017, Janus Kinase inhibitors (JAKi) were licensed for rheumatoid arthritis (RA) and Psoriatic arthritis PsA: Baricitinib and Tofacitinib. They are licensed for use in patients with high disease activity, with or without methotrexate, following failure of conventional synthetic Disease- Modifying Anti Rheumatic drugs (cs) DMARDs or of at least one biologic DMARD.
Aims/Background
To describe the baseline characteristics of patients attending the rheumatology department of University Hospital Waterford prescribed JAKi between July 2018 and March 2019.
Method
Information on Demographics, diagnosis, and adverse effects were collected for patients on JAKi.
Results
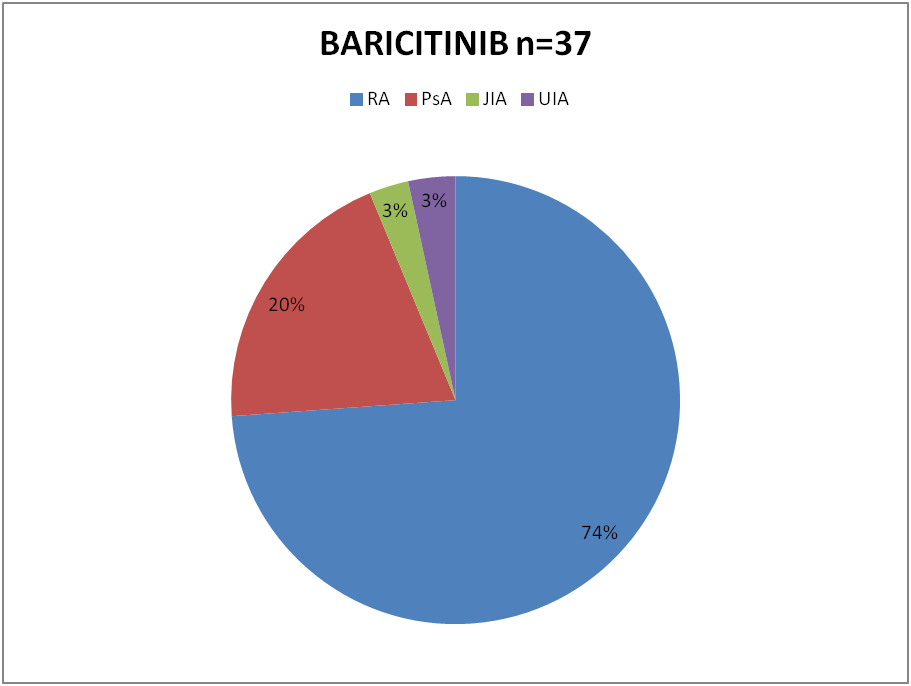
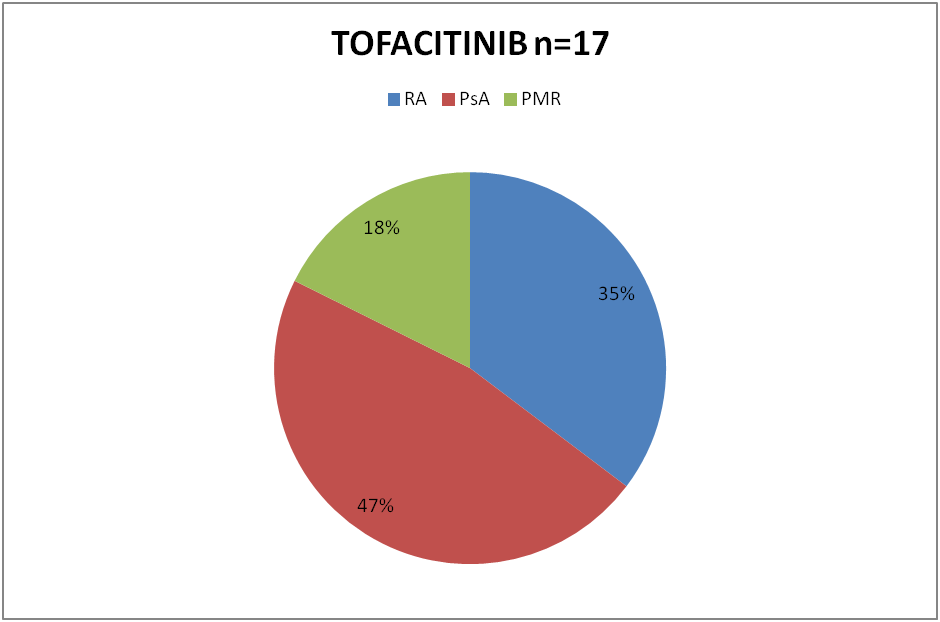
54 patients were started on JAKi during that period. 37 (68%) of them were started on Baricitinib and the remaining 17(32%) on Tofacitinib. Out of 37 patients on Baricitinib, 26 had diagnosis of Rheumatoid Arthritis (RA), 7 Psoriatic Arthritis (PsA), 1 Juvenile Inflammatory Arthritis (JIA) and 2 had Undifferentiated Inflammatory Arthritis. 6 of the 17 patients on Tofacitinib had RA, 8 had PsA and 3 patients Polymyalgia Rheumatica (PMR), (although not licensed), were started on Tofacitinib. 50 of the 54 patients had been on Methotrexate previously. 1 patient had contraindication to Methotrexate. 3 patients with PMR were tried on JAKi as steroid sparing agent. 22 patients were still on Methotrexate with JAKi. 36 patients had received various biologic DMARD with sub optimal response prior to treatment with JAKi. Varicella status was checked in all patients.
Baricitinib was stopped in 2 patients due to ineffectiveness. Both of these patients had advanced sero positive RA and failed multiple therapies. 2 other patients developed Shingles; Baricitinib was withdrawn in both. All others showed good clinical response.
Conclusions
To date, more patients have started baricitinib than tofacitinib in our department, possiby likely owing the once daily dosing. Two groups of patients are emerging; those receiving JAKi immediately following csDMARDS failure and those with established disease following multiple biologic DMARDs. Most of the patients in both groups showed good response to JAKi. A limitation of this review is that DAS scores were not recorded pre and post therapy for all patients. Long term follow up needed to see sustained effectiveness.