ISR Autumn Meeting 2018
Young Investigator Award

Dr Sarah Wade
18A127
Dysregulated miR-125a promotes joint angiogenesis through altered bioenergetics.
Author(s)
Sarah M. Wade1,2, Nils Ohnesorge2, Hayley Mc Loughlin1, Monika Biniecka2, Stephen Carter3, Michelle Trenkmann2, Trudy McGarry1,2, Mary Canavan1, Breandán Kennedy3, Douglas J. Veale2, Ursula Fearon1
Department(s)/Institutions
1 Molecular Rheumatology, School of Medicine, Trinity Biomedical Sciences Institute, Trinity College Dublin. 2 St. Vincent’s University Hospital, Dublin Academic Health Care, and University College Dublin. 3 Conway Institute, University College Dublin.
Introduction
Psoriatic arthritis (PsA) is characterised by an early vascular phase essential for pannus growth, immune responses and disease progression. Recently, numerous studies have highlighted the emerging importance of endothelial cell (EC) metabolism in disease.
Aims/Background
Herein, we propose microRNA, miR-125, modulates EC bioenergetics and orchestrates joint angiogenesis as characterised by ex-vivo associations, in-vitro assays and novel CRISPR/cas9 in-vivo zebrafish models.
Method
MiRNA levels were quantified in synovial tissue by RT-PCR and compared to macroscopic synovial vascularity and immunohistochemical analysis of angiogenic factors (FactorVIII/VEGF/ANG2). ECs (HMVEC) were transfected with anti-miR-125a for 24hr. Angiogenic mechanisms were quantified using tube formation assays, transwell matrigel chambers, wound repair and gene expression analysis. Real-time analysis of anti-125 treated ECs metabolism was assessed using the XF-24 Extracellular Flux Analyzer (Seahorse Bioscience). To determine if altered metabolism is observed ex vivo, metabolism markers (GAPDH/PKM2/GLUT1/ATP) were assessed by immunohistochemistry. In vivo, miR-125a CRISPR/Cas9-based knock-out zebrafish were generated and vascular development monitored. Finally, the therapeutic effect of blocking glycolysis using a small molecule, 3PO, which blocks PFKFB3, was assessed in miR-125a-/- ECs and zebrafish embryos.
Results
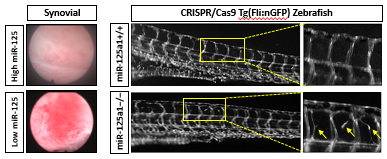
Synovial expression of miR-125 was significantly decreased in PsA versus OA synovial tissue, levels of which were associated with macroscopic and microscopic synovial vascularity. Decreased expression of miR-125a in HMVEC resulted in increased tube formation, invasion and migration properties. Inhibition of miR-125 promoted a metabolic shift towards glycolysis with parallel changes at the gene level. Ex vivo, increased vascular expression of glycolytic markers was observed in PsA versus OA synovial tissue. In vivo, miR-125a knockout zebrafish displayed increased vascular sprouting similar to the irregular nature of the vasculature within the PsA synovium. Finally, 3PO significantly inhibited anti-miR-125a-induced mechanism whilst normalising the vascular development of miR-125a-/- embryos.
Conclusions
Decreased expression of miR-125 in PsA synovium and in-vivo models was strongly associated pro-angiogenic mechanisms. Elevated glycolysis following miR-125 inhibition enabled ECs to meet the increased energy demands for new vessel formation. Correcting these miRNA deficiencies and their resulting metabolic shift, either by conventional pharmacological or as novel drug targets, may provide therapeutic benefit, especially in early disease.