19A117
Efficacy and Safety of Filgotinib for Patients with Rheumatoid Arthritis with Inadequate Response to Methotrexate: FINCH 1 Primary Outcome Results
Author(s)
Oliver Fitzgerald (1), Bernard G. Combe (2), Alan J. Kivitiz (3), Yoshiya Tanaka (4), Désirée van der Heijde (5), Franziska Matzkies (6), Beatrix Bartok (6), Lei Ye (6), Ying Guo (6), Chantal Tasset (7), John S. Sundy (6), Neelufar Mozaffarian (6), Robert B.M. Landewé (8), Sang-Cheol Bae (9), Edward C. Keystone (10), Peter Nash (11)
Department(s)/Institutions
1. University College Dublin, Dublin, Ireland, 2. CHU Montpellier, Montpellier University, Montpellier, France; 3. Altoona Center for Clinical Research, Duncansville, PA, USA; 4. University of Occupational and Environmental Health Japan, Kitakyushu, Japan; 5. Leiden University Medical Center, Leiden, Netherlands; 6. Gilead Sciences, Inc., Foster City, CA, USA; 7. Galapagos NV, Mechelen, Belgium; 8. Amsterdam University Medical Center, Amsterdam, Netherlands; 9. Hanyang University Hospital for Rheumatic Diseases, Seoul, Republic of Korea; 10. Mount Sinai Hospital and University of Toronto, Toronto, ON, Canada; 11. University of Queensland, St Lucia, Brisbane, Australia
Introduction
Filgotinib (FIL), an oral, selective Janus kinase 1 inhibitor, has shown efficacy and was well tolerated for rheumatoid arthritis (RA) treatment.
Aims/Background
To evaluate FIL’s efficacy and safety in patients with RA and inadequate response to methotrexate (MTX).
Method
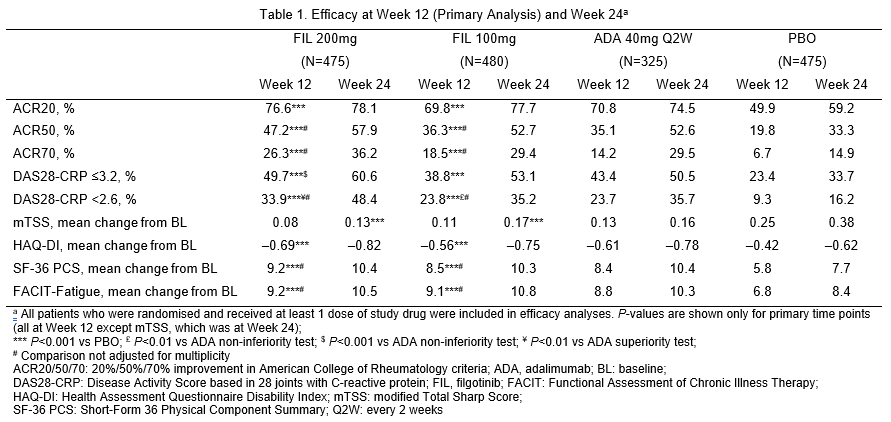
This Phase 3, double-blind, active- and placebo (PBO)-controlled study randomised patients with active RA (3:3:2:3) to FIL 200mg, FIL 100mg, adalimumab [ADA] 40mg every 2 weeks or PBO daily up to 52 weeks; results through Week 24 are presented. Patients also received MTX for ≥12 weeks with stable MTX dose for ≥4 weeks before study drug initiation. Primary endpoint was proportion achieving ACR20 response at Week 12; additional assessments were ACR50/70, DAS28-CRP ≤3.2 and 50% of ADA response]) was performed for DAS28-CRP ≤3.2 and <2.6.
Results
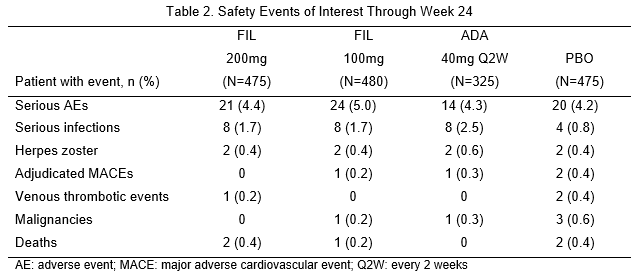
1,755 patients received the study drug, with 475 FIL 200mg; 480 FIL 100mg; 325 ADA; and 475 PBO. 81.8% were female, mean (standard deviation [SD]) RA duration 7.8 (7.6) years and mean (SD) DAS28-CRP 5.7 (0.9). At Week 12, significantly more patients on FIL 200mg and 100mg achieved ACR20 response compared with PBO (Table 1). Compared with PBO, more patients receiving FIL achieved ACR50/70 responses, DAS28-CRP ≤3.2 and <2.6, had lower radiographic progression and PROs improvements (Table 1). Non-inferiority of FIL 200mg to ADA was met on DAS28-CRP ≤3.2. FIL safety profile was consistent with prior studies through Week 24 (Table 2).
Conclusions
FIL 200mg and 100mg led to significant improvement in signs and symptoms of RA, prevented radiographic progression and improved physical function compared with PBO, and was well tolerated among patients with RA with inadequate response to MTX. Efficacy of FIL 200mg was non-inferior to ADA based on DAS28-CRP ≤3.2.