18A150
Long-term outcome of rituximab in rheumatoid arthritis: real world experience
Author(s)
Candice Low, Richard Conway, Francis Young, Eamonn S Molloy, Anne Barbara Mongey, Oliver FitzGerald, Gerry Wilson, Ursula Fearon, Douglas Veale
Department(s)/Institutions
Centre for Arthritis and Rheumatic Disease, St. Vincent’s University Hospital, University College Dublin, Ireland, and Department of Molecular Rheumatology, School of Medicine, Trinity Biomedical Sciences Institute, Trinity College Dublin, Dublin 2, Ireland
Introduction
Rituximab is an effective treatment for rheumatoid arthritis (RA). Data on long-term outcomes following rituximab treatment are limited.
Aims/Background
To evaluate the long-term efficacy of, and identify predictors of response to, rituximab in our centre.
Method
We conducted an observational study of RA patients treated with rituximab from 2003-2016. Demographic and clinical characteristics, including response to treatment, were assessed with tender joint count, swollen joint count, ESR, and CRP. Arthroscopy was performed in patients where clinically indicated. Univariate and multivariable logistic regression models were established to evaluate baseline predictors of treatment response. Remission was defined as DAS28-CRP <2.6 or meeting the 2011 ACR/EULAR remission criteria.
Results
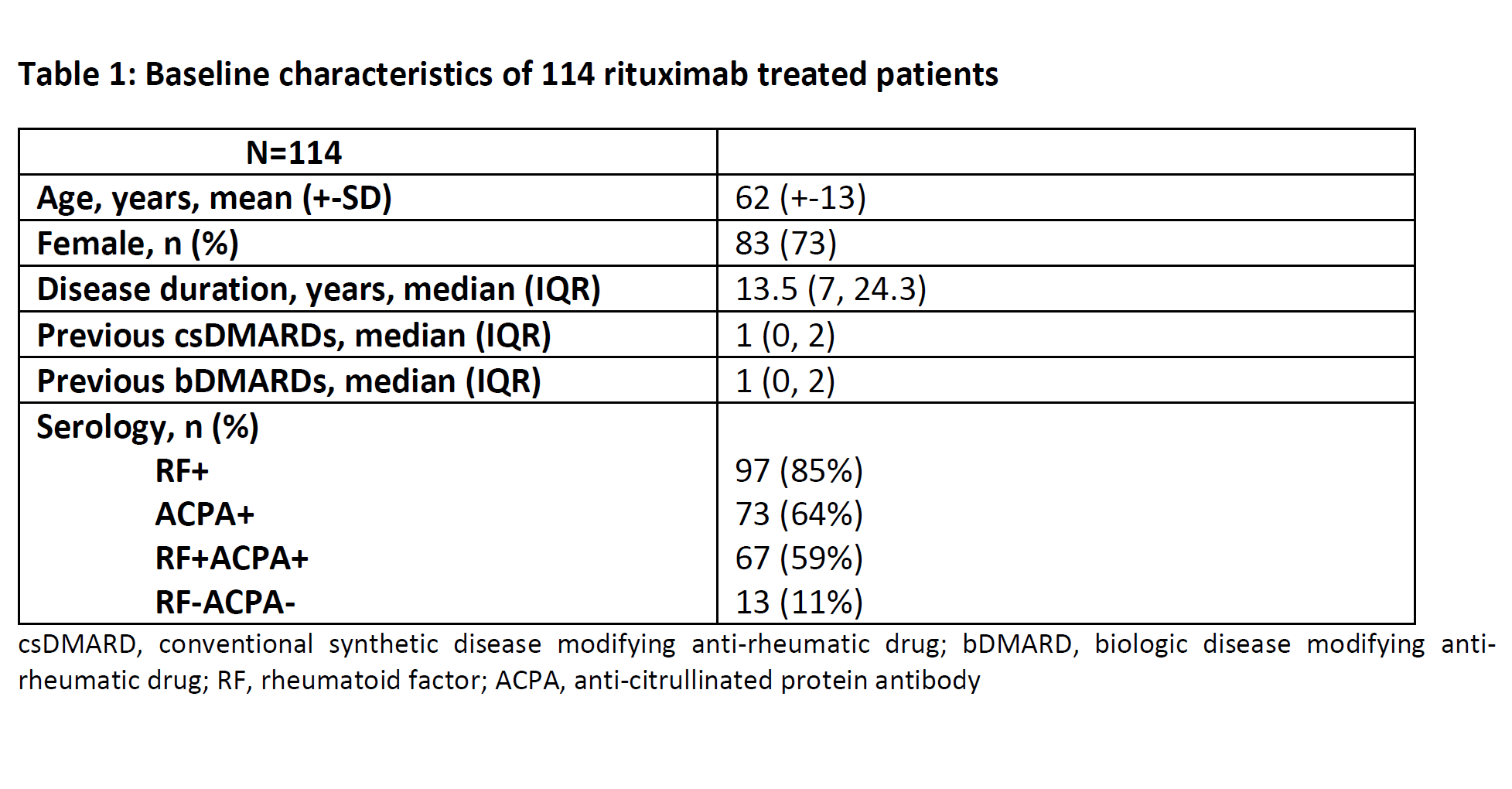
114 RA patients were treated with rituximab. Baseline characteristics of patients are shown in Table 1. 34 were receiving rituximab monotherapy, 80 were receiving combination therapy with a csDMARD. At last follow-up median (IQR) duration of rituximab treatment was 3.1 (1.8, 6.1) years. 68 (60%) patients maintained remission, 14 (12%) were primary non-responders (7% RF-, 50% ACPA-, 7% RF-ACPA-), 25 (22%) secondary non-responders (24% RF-, 40% ACPA-, 12% RF-ACPA-), and 7 (6%) stopped rituximab due to adverse events (3 hypersensitivity reactions, 2 recurrent LRTIs, 1 neutropenia, 1 severe herpes zoster). Of the 68 patients in remission, 26 (38%) were on rituximab monotherapy and 42 (62%) were receiving combination therapy with a csDMARD. Of the 39 biologic naïve patients, 24 (62%) were in remission and 15 (38%) were not; rituximab achieved equally good outcomes in patients who had previously failed a biologic. Outcomes are shown in Table 2. No significant baseline predictors of treatment response were identified using logistic regression modelling. In the 44 patients who had an arthroscopy, baseline ESR (p=0.312), CRP (p=0.590), patient global assessment (p=0.934), DAS28-CRP (p=1), TJC (p=0.750), SJC (p=0.848), macroscopic synovitis (p=0.490), macroscopic vascularity (p=0.936), and histologic inflammation (p=0.146) did not predict response to rituximab.
Conclusions
Rituximab is an effective long-term treatment, with 60% remission, for many of our RA patients, including those who have previously failed a biologic. In this cohort, no baseline demographic, clinical, or serological characteristics accurately predict response to rituximab.