18A105
Real Life Switching from Infliximab Innovator (Remicade) to Biosimilar (Inflectra) in Patients with Various Rheumatic Diseases: a 6-month Single-Centre Prospective Observational Study
Author(s)
Aqeel M. Anjum 1,2 W. L. Ng1,2, A. Sebastian1, 2, M. Brady1, 2, E. Fitzgerald2, B. McCarthy2, M. Gillespie1, J. Devlin1, 2, A. Fraser1, 2
Department(s)/Institutions
1. Rheumatology, University Hospitals Limerick, 2. Rheumatology, Croom Orthopedic Hospital, Limerick, Ireland.
Introduction
Inflectra, biosimilar infliximab has been approved by the European Medicine Agency since September 2013 for all licensed indications of Remicade (innovator infliximab) having demonstrated similar pharmacokinetics, safety, and efficacy to those of innovator INX. Although Biosimilars can offer significant cost savings, there is a paucity of real-world data and guidelines regarding switching from innovator Remicade to Inflectra.
Aims/Background
The aim of this study was to explore the efficacy, safety, acceptance and retention rate of biosimilar CT-P13 after switching from Remicade, originator infliximab in patients with various rheumatic diseases.
Method
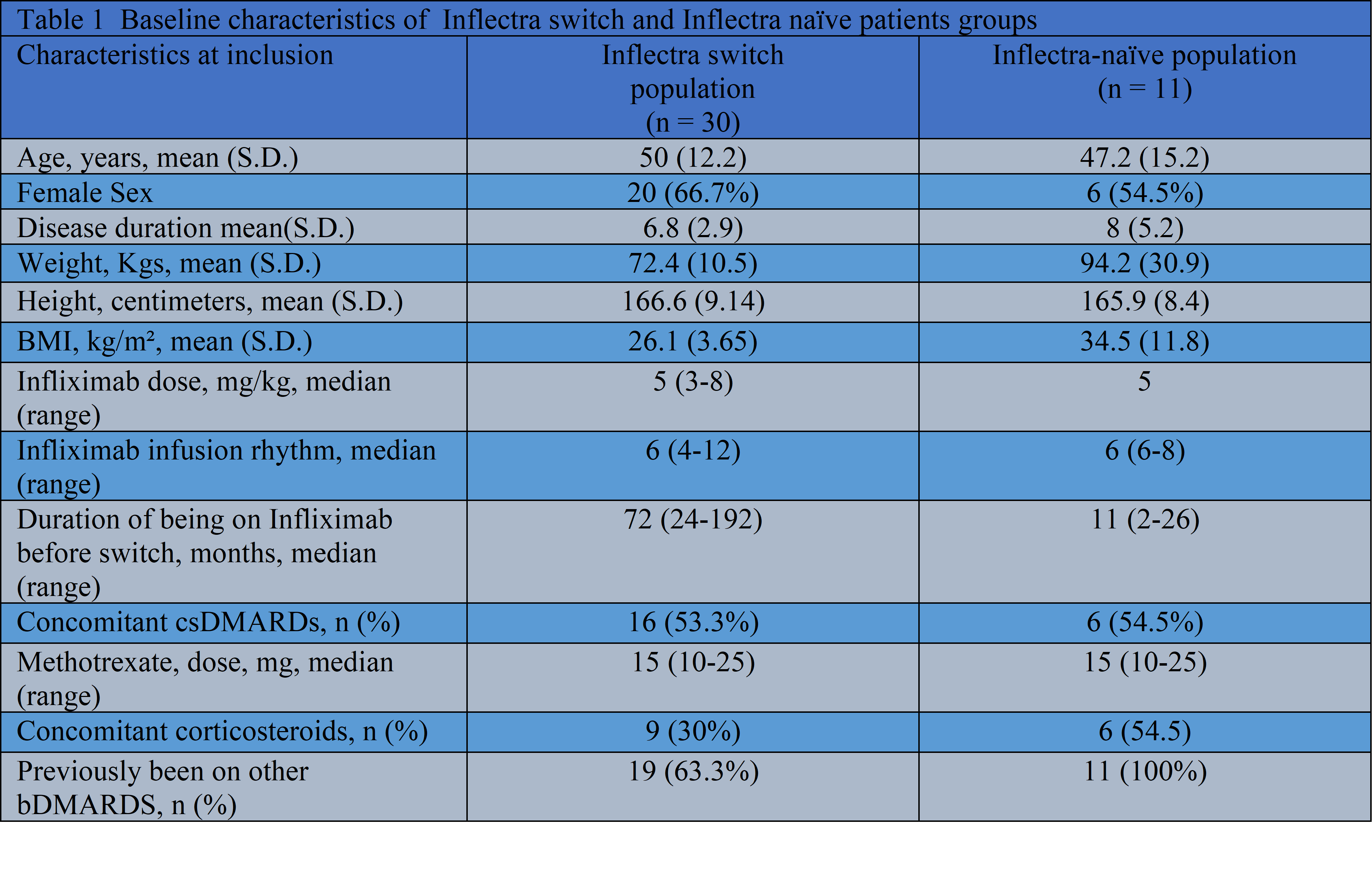
A proposal to switch was made to all patients attending our rheumatology infusion unit. Baseline demographics and clinical characteristics were obtained before switching to Inflectra (biosimilar). Disease activity and safety assessment were undertaken before and then every 12 weeks after switching. The retention rate of Inflectra switch patients was compared with a cohort of Inflectra naive (11 patients) and historic Remicade (31 patients) patients.
Results
: Thirty out of thirty-one patients {median (IQR) age 50 (18), 20F} with various rheumatic diseases (9 with diagnosis of AS, 6 with RA, 6 with Behcet disease, 3 with Enteropathic arthritis, 2 with psoriatic arthritis and 1 with JIA, Graves ophthalmopathy, juvenile dermatomyositis and undifferentiated inflammatory arthritis each) agreed to the switch. After 6 months of Inflectra, we could not find any statistical difference in term of mean values of PGA {33 (26.3) vs 35.3 (24) p=0.37}, BASDAI (3.12 (1.2) vs 2.98 (1.5) p=0.60} , SDAI {14.6 (16.5) vs 13.1 (10.4) p=0.65}, DAS28CRP {3.9 (1.6) vs 3.28 (1.0) p=0.85}, CRP {3.13 (4.2) vs 3.48 (4.8) p=0.09} , Behcet disease score {1.17 (1.3) vs 1.33 (2.16) p=0.77} and HAQ-DI {0.42 (0.45) vs 0.45 (0.47) p=0.18}. The retention rate on Inflectra switch was 86.7% as compared to 90.9% on Inflectra naive cohort and 100% for historic Remicade cohort.
Conclusions
These results demonstrate that is Inflectra is comparable to Remicade in efficacy and there are no new safety signals. Subjective symptoms were an important cause for a slightly lower retention rate in switch group and this we believe may be due to a degree of the nocebo effect.