18A177
Role of Macrophage Migration Inhibitory Factor in Rheumatoid and Psoriatic Arthritis
Author(s)
Tatsiana Y. Rakovich, Clare Cunningham, Sharon Ansboro, Trudy McGarry, Douglas J. Veale, Ursula Fearon
Department(s)/Institutions
The Department of Molecular Rheumatology, Trinity College Dublin, Ireland. The Centre for Arthritis and Rheumatic Disease, Dublin Academic Medical Centre, St. Vincent's University Hospital, Elm Park, Dublin 4, Ireland.
Introduction
Macrophage migration inhibitory factor (MIF) is a key regulator of pro-inflammatory cytokines and has been implicated in angiogenesis and pathogenesis of several diseases such as rheumatoid arthritis (RA). Synovial fibroblasts (SF) and macrophages are considered to be key players in the hyperplastic synovial tissue that invades and degrades adjacent cartilage and bone in patients with inflammatory arthritis.
Aims/Background
To perform a comparative analysis of the expression of MIF, its regulation and pathogenic roles in patients with RA, Psoriatic Arthritis (PsA), Osteoarthritis (OA) and in Arthralgia patients (pre-RA) in vitro and ex vivo.
Method
MIF expression was quantified in RA, PsA, OA and arthralgia synovial tissue sections by immunohistology, and real-time PCR. Peripheral blood mononuclear cells (PBMC) were isolated from healthy donors, and patients with OA, RA, PsA and Arthralgia and primary macrophages (Mf) differentiated from isolated CD14+ monocytes and polarised into M1 and M2 phenotypes. Primary synovial fibroblasts (SFC) from OA, RA and PsA patients were cultured with or without TNFa (10ng/mL). PBMC, Mf, SFC and explant mRNA was isolated and MIF gene expression evaluated by RT-PCR. Mf and SFC supernatants were harvested and assayed for soluble MIF by ELISA. Human endothelial (HUVEC) cells and Mf were cultured with recombinant MIF protein pro-inflammatory/angiogenic markers quantified in supernatants by ELISA and cell lysates by RT-PCR. GraphPad Prism Ver7 was used for statistical analysis.
Results
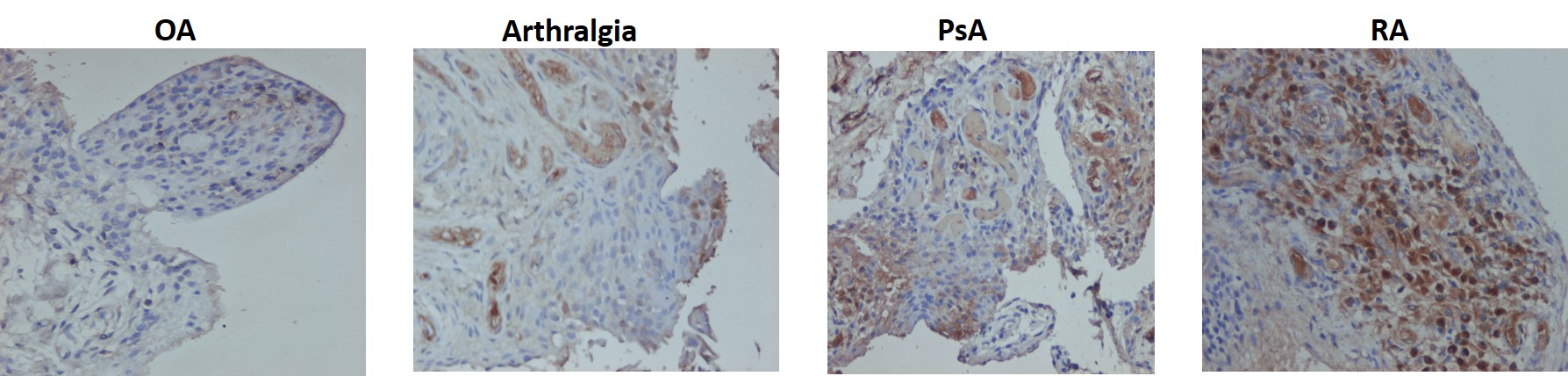
MIF protein expression was increased in RA and PsA synovial tissue compared to OA and arthralgia. MIF mRNA was higher in RA vs PsA and OA. MIF mRNA expression was increased in Mfs from RA patients in comparison to healthy controls. MIF mRNA expression levels were found to be higher in RA SFC in comparison to PsA SFC patients. Interestingly, treatment with TNF-a resulted in decreased levels of MIF both in RA and PsA SFC. Addition of rhMIF activated pro-inflammatory responses (IL-1b,-IL-6,-IL8,-MCP-1,-GAPDH,-Notch-1 of healthy unpolarised macrophages. However, rhMIF had no effect on the pro-inflammatory and angiogenic markers in HUVEC endothelial cells.
Conclusions
MIF may have a key role in promoting the pathogenesis of RA and has a good potential as a therapeutic target for RA.